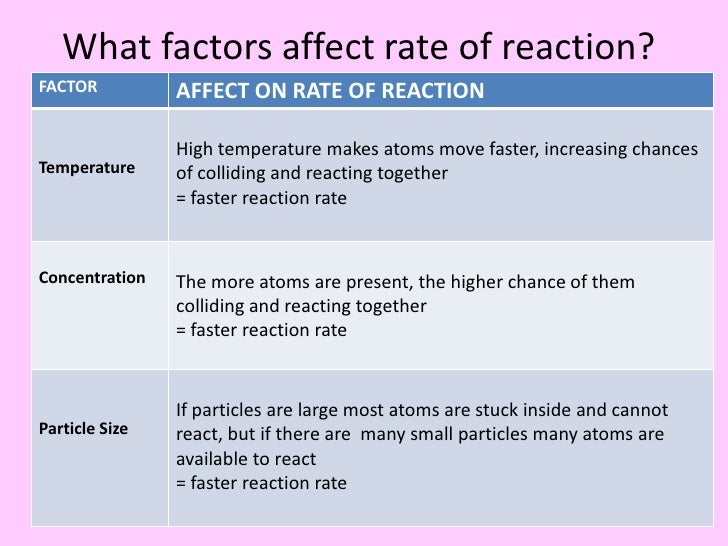
What Is Meant By Rate Chemical Reaction . The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. Increasing the concentration of either reactant increases the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. The rate of reaction is proportional to the number of collisions over time; Derive rate expressions from the balanced equation for a given chemical reaction;
from pt.slideshare.net
It is often expressed in terms of either the concentration (amount per unit volume) of a product that. The rate of reaction is proportional to the number of collisions over time; The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. Derive rate expressions from the balanced equation for a given chemical reaction; It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. Increasing the concentration of either reactant increases the. The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds.
Rates of chemical reactions
What Is Meant By Rate Chemical Reaction It is expressed as the concentration of the reactants consumed or products formed per unit time. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction is proportional to the number of collisions over time; Increasing the concentration of either reactant increases the. Derive rate expressions from the balanced equation for a given chemical reaction; The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant.
From www.researchgate.net
Reaction rate equation Download Table What Is Meant By Rate Chemical Reaction The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. It is often expressed in. What Is Meant By Rate Chemical Reaction.
From owlcation.com
Chemical Reactions and Chemical Equations Owlcation What Is Meant By Rate Chemical Reaction Derive rate expressions from the balanced equation for a given chemical reaction; The rate of reaction is proportional to the number of collisions over time; The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. Reaction. What Is Meant By Rate Chemical Reaction.
From www.slideshare.net
Rates of chemical reactions What Is Meant By Rate Chemical Reaction The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant.. What Is Meant By Rate Chemical Reaction.
From marshall-has-roth.blogspot.com
What is Rate of Reaction MarshallhasRoth What Is Meant By Rate Chemical Reaction It is often expressed in terms of either the concentration (amount per unit volume) of a product that. The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. Reaction rate, in. What Is Meant By Rate Chemical Reaction.
From db-excel.com
Rates Chemical Reactions Reaction Product Calculator Science — What Is Meant By Rate Chemical Reaction Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction is proportional to the number of collisions over time; The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants. What Is Meant By Rate Chemical Reaction.
From www.youtube.com
Chemical 2 Average Rate of Reaction Instantaneous Rate What Is Meant By Rate Chemical Reaction Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The reaction rate for a given chemical reaction is the measure of the change in concentration. What Is Meant By Rate Chemical Reaction.
From en.ppt-online.org
Rates of reaction online presentation What Is Meant By Rate Chemical Reaction The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Increasing the concentration of either reactant increases the. It is often expressed in terms of either the concentration (amount per unit volume) of. What Is Meant By Rate Chemical Reaction.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions What Is Meant By Rate Chemical Reaction The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. It is often expressed in terms of either the concentration (amount per unit volume) of a product that. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. It is expressed. What Is Meant By Rate Chemical Reaction.
From studylib.net
The Rate of Chemical Reactions What Is Meant By Rate Chemical Reaction It is often expressed in terms of either the concentration (amount per unit volume) of a product that. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. Reaction rate, in chemistry, the speed at which a chemical. What Is Meant By Rate Chemical Reaction.
From quizlet.com
4 Main factors that affect the rate of chemical reactions Diagram Quizlet What Is Meant By Rate Chemical Reaction It is often expressed in terms of either the concentration (amount per unit volume) of a product that. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The reaction rate. What Is Meant By Rate Chemical Reaction.
From www.slideshare.net
Rates of reaction What Is Meant By Rate Chemical Reaction Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction is proportional to the number of collisions. What Is Meant By Rate Chemical Reaction.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6516140 What Is Meant By Rate Chemical Reaction Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. Derive rate expressions from the balanced equation for a given chemical reaction; The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The reaction rate for a given chemical reaction is the measure of the change in. What Is Meant By Rate Chemical Reaction.
From www.studocu.com
Chemical Reaction Rates Chemical Reaction Rates What factors affect What Is Meant By Rate Chemical Reaction The reaction rate for a given chemical reaction is the measure of the change in concentration of the reactants or the. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. The reaction rate or rate of reaction is the speed at. What Is Meant By Rate Chemical Reaction.
From www.slideserve.com
PPT Rate of reaction definition PowerPoint Presentation, free What Is Meant By Rate Chemical Reaction It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. Reaction rate, in chemistry, the. What Is Meant By Rate Chemical Reaction.
From slideplayer.com
Reaction Rates. ppt download What Is Meant By Rate Chemical Reaction The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. It is expressed as the concentration of the reactants consumed or products formed per unit time. Increasing the concentration of either reactant increases the. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate. What Is Meant By Rate Chemical Reaction.
From eyedap.pics
Chemical Reaction Rates Chemistry for Majors (2023) What Is Meant By Rate Chemical Reaction Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. Increasing the concentration of either reactant increases the. The. What Is Meant By Rate Chemical Reaction.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical What Is Meant By Rate Chemical Reaction The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction. It is often expressed in terms of either the concentration (amount per unit volume) of a product that. Derive rate expressions from the balanced equation for a given chemical reaction; Increasing the concentration of either reactant increases the. The. What Is Meant By Rate Chemical Reaction.
From www.expii.com
What Is a Chemical Reaction? — Overview & Examples Expii What Is Meant By Rate Chemical Reaction The rate of reaction is proportional to the number of collisions over time; Increasing the concentration of either reactant increases the. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. Derive rate expressions from the balanced equation for a given chemical reaction; The rate of reaction refers to the speed at which the products are formed from. What Is Meant By Rate Chemical Reaction.